What's the best battery?
Battery novices often brag about miracle batteries that offer very high energy densities, deliver 1000 charge/discharge cycles and are paper-thin. These attributes are indeed achievable but not on one and the same battery pack.
A certain battery may be designed for small size and long runtime but has a limited cycle life. Another pack may be built for durability and is big and bulky. A third may have high energy density and long durability but is made for a special application and is too expensive for the average consumer. A lithium-based battery can be designed for maximum energy density but its safety would be compromised.
Battery manufacturers are aware of customer needs and offer packs that best suit the application. The mobile phone industry is a good example of this clever adaptation. Here, small size and high energy density reign in favor of longevity. Short service life is not an issue because a device is often replaced before the battery is worn out.
Below is a summary of the strength and limitations of today's popular battery systems. Although energy density is paramount, other important attributes are service life, load characteristics, maintenance requirements, self-discharge costs and safety. Nickel-cadmium is the first rechargeable battery in small format and forms a standard against which other chemistries are commonly compared. The trend is towards lithium-based systems.
Nickel-cadmium - mature but has moderate energy density. Nickel-cadmium is used where long life, high discharge rate and extended temperature range is important. Main applications are two-way radios, biomedical equipment and power tools. Nickel-cadmium contains toxic metals.
Nickel-metal-hydride - has a higher energy density compared to nickel-cadmium at the expense of reduced cycle life. There are no toxic metals. Applications include mobile phones and laptop computers. NiMH is viewed as steppingstone to lithium-based systems.
Lead-acid - most economical for larger power applications where weight is of little concern. Lead-acid is the preferred choice for hospital equipment, wheelchairs, emergency lighting and UPS systems. Lead acid is inexpensive and rugged. It serves a unique niche that would be hard to replace with other systems.
Lithium-ion - fastest growing battery system; offers high-energy density and low weight. Protection circuit are needed to limit voltage and current for safety reasons. Applications include notebook computers and cell phones. High current versions are available for power tools and medical devices.
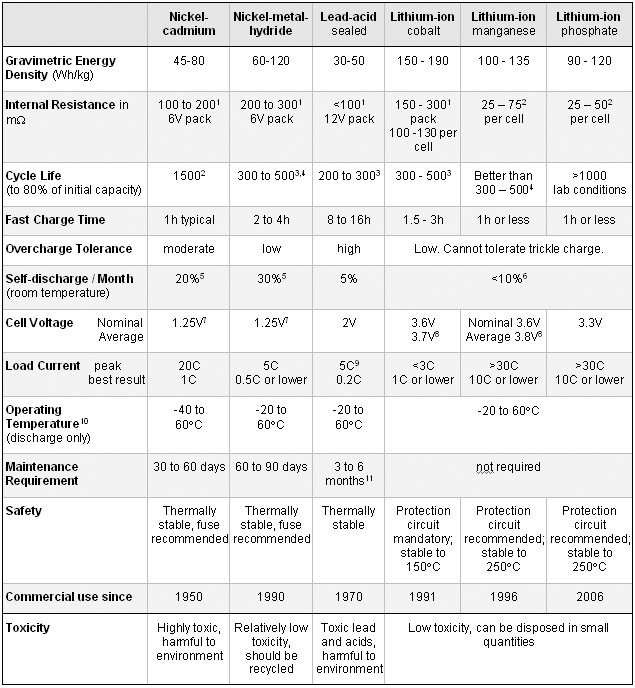
Table 1 summarizes the characteristics of the common batteries. The figures are based on average ratings at time of publication. Lithium-ion is divided into three versions: The traditional cobalt that is commonly used in cell phones, cameras and laptops; the manganese (spinel) that power high-end power tools and the new phosphate that competes head-on with spinel. Lithium-ion polymer is not listed as a separate system. Its unique construction performs in a same way to cobalt-based lithium-ion.
Table 1: Characteristics of commonly used rechargeable batteries.
1) Internal resistance of a battery pack varies with mAh rating, wiring and number of cells. Protection circuit of lithium-ion adds about 100mW.
2) Based on 18650 cell size. Cell size and design determines internal resistance. Larger cells can have an impedance of <15mOhms,
3) Cycle life is based on battery receiving regular maintenance. Failing to apply periodic full discharge cycles may reduce the cycle life by a factor of three.
4) Cycle life is based on the depth of discharge. Shallow discharges provide more cycles than deep discharges.
5) The self-discharge is highest immediately after charge, and then tapers off. The capacity loss of nickel-cadmium is 10% in the first 24h, then declines to about 10% every 30 days thereafter. High temperature increases self-discharge.
6) Internal protection circuits typically consume 3% of the stored energy per month.
7) The traditional nominal voltage is 1.25V; 1.2V is more commonly used to harmonize with lithium-ion (3 in series = 3.6V).
8) Lithium-ion is often rated higher than the nominal 3.6V. Based on average voltage under load.
9) Capable of high current pulses; needs time to recuperate.
10) Applies to discharge only; charge temperature range is more confined. Delivers lower capacity at lower temperatures.
11) Maintenance may be in the form of 'equalizing' or 'topping' charge to prevent sulphation.
In subsequent columns I will describe the strength and limitation of each chemistry in more detail. We will examine charging techniques and explore methods to get the most of these batteries.
The nickel-based battery, its dominance and the future
In this section we evaluate the strengths and limitations of various battery chemistries, beginning with the nickel. Each battery system offers unique advantages but none provides a fully satisfactory solution. With the increased selection of battery chemistries available today, better choices can be made to address specific battery needs. A careful evaluation of each battery's attribute is important. Because of similarities, both nickel-cadmium and nickel-metal hydride are covered in this paper.
The nickel-cadmium battery
Swedish Waldmar Jungner invented the nickel-cadmium battery in 1899. At that time, the materials were expensive compared to other battery types available and its use was limited to special applications. In 1932, the active materials were deposited inside a porous nickel-plated electrode and in 1947 research began on a sealed nickel-cadmium battery.
Rather than venting, the internal gases generated during charge were recombined. These advances led to the modern sealed nickel-cadmium battery, which is in use today.
Nickel-cadmium prefers fast charge to slow charge and pulse charge to DC charge. It is a strong and silent worker; hard labor poses little problem. In fact, nickel-cadmium is the only battery type that performs well under rigorous working conditions. All other chemistries prefer a shallow discharge and moderate load currents.
Nickel-cadmium does not like to be pampered by sitting in chargers for days and being used only occasionally for brief periods. A periodic full discharge is so important that, if omitted, large crystals will form on the cell plates (also referred to as memory) and the nickel-cadmium will gradually lose its performance.
Among rechargeable batteries, nickel-cadmium remains a popular choice for two-way radios, emergency medical equipment and power tools. There is shift towards batteries with higher energy densities and less toxic metals but alternative chemistries cannot always match the superior durability and low cost of nickel-cadmium.
Here is a summary of the advantages and limitations of nickel-cadmium batteries.
Advantages
- Fast and simple charge, even after prolonged storage.
- High number of charge/discharge cycles - if properly maintained, nickel-cadmium provides over 1000 charge/discharge cycles.
- Good load performance - nickel-cadmium allows recharging at low temperatures.
- Long shelf life - five-year storage is possible. Some priming prior to use will be required.
- Simple storage and transportation - most airfreight companies accept nickel-cadmium without special conditions.
- Good low temperature performance.
- Forgiving if abused - nickel-cadmium is one of the most rugged rechargeable batteries.
- Economically priced - nickel-cadmium is lowest in terms of cost per cycle.
- Available in a wide range of sizes and performance options - most nickel-cadmium cells are cylindrical.
Limitations
- Relatively low energy density.
- Memory effect - nickel-cadmium must periodically be exercised (discharge/charge) to prevent memory.
- Environmentally unfriendly - nickel-cadmium contains toxic metals. Some countries restrict its use.
- Relatively high self-discharge - needs recharging after storage
The nickel-metal-hydride battery
Research on the nickel-metal-hydride system started in the 1970s as a means of storing hydrogen for the nickel hydrogen battery. Today, nickel hydrogen is used mainly for satellite applications. nickel hydrogen batteries are bulky, require high-pressure steel canisters and cost thousands of dollars per cell.
In the early experimental days of nickel-metal hydride, the metal hydride alloys were unstable in the cell environment and the desired performance characteristics could not be achieved. As a result, the development of nickel-metal hydride slowed down. New hydride alloys were developed in the 1980s that were stable enough for use in a cell. Since then, nickel-metal hydride has steadily improved.
The success of nickel-metal hydride has been driven by high energy density and the use of environmentally friendly metals. The modern nickel-metal hydride offers up to 40% higher energy density compared to the standard nickel-cadmium. There is potential for yet higher capacities, but not without some negative side effects.
Nickel-metal hydride is less durable than nickel-cadmium. Cycling under heavy load and storage at high temperature reduces the service life. nickel-metal hydride suffers from high self-discharge, which is higher than that of nickel-cadmium.
Nickel-metal hydride has been replacing nickel-cadmium in markets such as wireless communications and mobile computing. Experts agree that nickel-metal hydride has greatly improved over the years, but limitations remain. Most shortcomings are native to the nickel-based technology and are shared with nickel-cadmium. It is widely accepted that nickel-metal hydride is an interim step to lithium-based battery technology.
Here is a summary of the advantages and limitations of nickel-metal hydride batteries.
Advantages
- 30-40% higher capacity than standard nickel-cadmium. Nickel-metal-hydride has potential for yet higher energy densities.
- Less prone to memory than nickel-cadmium - fewer exercise cycles are required.
- Simple storage and transportation - transport is not subject to regulatory control.
- Environmentally friendly - contains only mild toxins; profitable for recycling.
Limitations
- Limited service life - the performance starts to deteriorate after 200-300 cycles if repeatedly deeply cycled.
- Relatively short storage of three years. Cool temperature and a partial charge slows aging.
- Limited discharge current - although nickel-metal-hydride is capable of delivering high discharge currents, heavy load reduces the battery's cycle life.
- More complex charge algorithm needed - nickel-metal-hydride generates more heat during charge and requires slightly longer charge times than nickel-cadmium. Trickle charge settings are critical because the battery cannot absorb overcharge.
- High self-discharge - typically 50% higher than nickel-cadmium.
- Performance degrades if stored at elevated temperatures - nickel-metal-hydride should be stored in a cool place at 40% state-of-charge.
- High maintenance - nickel-metal hydride requires regular full discharge to prevent crystalline formation. nickel-cadmium should be exercised once a month, nickel-metal-hydride once in every 3 months.
_________________________
Is lithium-ion the ideal battery?
For many years, nickel-cadmium had been the only suitable battery for portable equipment from wireless communications to mobile computing. Nickel-metal-hydride and lithium-ion emerged In the early 1990s, fighting nose-to-nose to gain customer's acceptance. Today, lithium-ion is the fastest growing and most promising battery chemistry.
The lithium-ion battery
Pioneer work with the lithium battery began in 1912 under G.N. Lewis but it was not until the early 1970s when the first non-rechargeable lithium batteries became commercially available. lithium is the lightest of all metals, has the greatest electrochemical potential and provides the largest energy density for weight.
Attempts to develop rechargeable lithium batteries failed due to safety problems. Because of the inherent instability of lithium metal, especially during charging, research shifted to a non-metallic lithium battery using lithium ions. Although slightly lower in energy density than lithium metal, lithium-ion is safe, provided certain precautions are met when charging and discharging. In 1991, the Sony Corporation commercialized the first lithium-ion battery. Other manufacturers followed suit.
The energy density of lithium-ion is typically twice that of the standard nickel-cadmium. There is potential for higher energy densities. The load characteristics are reasonably good and behave similarly to nickel-cadmium in terms of discharge. The high cell voltage of 3.6 volts allows battery pack designs with only one cell. Most of today's mobile phones run on a single cell. A nickel-based pack would require three 1.2-volt cells connected in series.
Lithium-ion is a low maintenance battery, an advantage that most other chemistries cannot claim. There is no memory and no scheduled cycling is required to prolong the battery's life. In addition, the self-discharge is less than half compared to nickel-cadmium, making lithium-ion well suited for modern fuel gauge applications. lithium-ion cells cause little harm when disposed.
Despite its overall advantages, lithium-ion has its drawbacks. It is fragile and requires a protection circuit to maintain safe operation. Built into each pack, the protection circuit limits the peak voltage of each cell during charge and prevents the cell voltage from dropping too low on discharge. In addition, the cell temperature is monitored to prevent temperature extremes. The maximum charge and discharge current on most packs are is limited to between 1C and 2C. With these precautions in place, the possibility of metallic lithium plating occurring due to overcharge is virtually eliminated.
Aging is a concern with most lithium-ion batteries and many manufacturers remain silent about this issue. Some capacity deterioration is noticeable after one year, whether the battery is in use or not. The battery frequently fails after two or three years. It should be noted that other chemistries also have age-related degenerative effects. This is especially true for nickel-metal-hydride if exposed to high ambient temperatures. At the same time, lithium-ion packs are known to have served for five years in some applications.
Manufacturers are constantly improving lithium-ion. New and enhanced chemical combinations are introduced every six months or so. With such rapid progress, it is difficult to assess how well the revised battery will age.
Storage in a cool place slows the aging process of lithium-ion (and other chemistries). Manufacturers recommend storage temperatures of 15°C (59°F). In addition, the battery should be partially charged during storage. The manufacturer recommends a 40% charge.
The most economical lithium-ion battery in terms of cost-to-energy ratio is the cylindrical 18650 (18 is the diameter and 650 the length in mm). This cell is used for mobile computing and other applications that do not demand ultra-thin geometry. If a slim pack is required, the prismatic lithium-ion cell is the best choice. These cells come at a higher cost in terms of stored energy.
Advantages
- High energy density - potential for yet higher capacities.
- Does not need prolonged priming when new. one regular charge is all that's needed.
- Relatively low self-discharge - self-discharge is less than half that of nickel-based batteries.
- Low Maintenance - no periodic discharge is needed; there is no memory.
- Specialty cells can provide very high current to applications such as power tools.
Limitations
- Requires protection circuit to maintain voltage and current within safe limits.
- Subject to aging, even if not in use - storage in a cool place at 40% charge reduces the aging effect.
- Transportation restrictions - shipment of larger quantities may be subject to regulatory control. This restriction does not apply to personal carry-on batteries. (See last section)
- Expensive to manufacture - about 40 percent higher in cost than nickel-cadmium.
- Not fully mature - metals and chemicals are changing on a continuing basis.
The lithium Polymer battery
The lithium-polymer differentiates itself from conventional battery systems in the type of electrolyte used. The original design, dating back to the 1970s, uses a dry solid polymer electrolyte. This electrolyte resembles a plastic-like film that does not conduct electricity but allows ions exchange (electrically charged atoms or groups of atoms). The polymer electrolyte replaces the traditional porous separator, which is soaked with electrolyte.
The dry polymer design offers simplifications with respect to fabrication, ruggedness, safety and thin-profile geometry. With a cell thickness measuring as little as one millimeter (0.039 inches), equipment designers are left to their own imagination in terms of form, shape and size.
Unfortunately, the dry lithium-polymer suffers from poor conductivity. The internal resistance is too high and cannot deliver the current bursts needed to power modern communication devices and spin up the hard drives of mobile computing equipment. Heating the cell to 60°C (140°F) and higher increases the conductivity, a requirement that is unsuitable for portable applications.
To compromise, some gelled electrolyte has been added. The commercial cells use a separator/ electrolyte membrane prepared from the same traditional porous polyethylene or polypropylene separator filled with a polymer, which gels upon filling with the liquid electrolyte. Thus the commercial lithium-ion polymer cells are very similar in chemistry and materials to their liquid electrolyte counter parts.
Lithium-ion-polymer has not caught on as quickly as some analysts had expected. Its superiority to other systems and low manufacturing costs has not been realized. No improvements in capacity gains are achieved - in fact, the capacity is slightly less than that of the standard lithium-ion battery. Lithium-ion-polymer finds its market niche in wafer-thin geometries, such as batteries for credit cards and other such applications.
Advantages
- Very low profile - batteries resembling the profile of a credit card are feasible.
- Flexible form factor - manufacturers are not bound by standard cell formats. With high volume, any reasonable size can be produced economically.
- Lightweight - gelled electrolytes enable simplified packaging by eliminating the metal shell.
- Improved safety - more resistant to overcharge; less chance for electrolyte leakage.
Limitations
- Lower energy density and decreased cycle count compared to lithium-ion.
- Expensive to manufacture.
- No standard sizes. Most cells are produced for high volume consumer markets.
- Higher cost-to-energy ratio than lithium-ion
Restrictions on lithium content for air travel
Air travelers ask the question, "How much lithium in a battery am I allowed to bring on board?" We differentiate between two battery types: Lithium metal and lithium-ion.
Most lithium metal batteries are non-rechargeable and are used in film cameras. Lithium-ion packs are rechargeable and power laptops, cellular phones and camcorders. Both battery types, including spare packs, are allowed as carry-on but cannot exceed the following lithium content:
- 2 grams for lithium metal or lithium alloy batteries
- 8 grams for lithium-ion batteries
Lithium-ion batteries exceeding 8 grams but no more than 25 grams may be carried in carry-on baggage if individually protected to prevent short circuits and are limited to two spare batteries per person.
How do I know the lithium content of a lithium-ion battery? From a theoretical perspective, there is no metallic lithium in a typical lithium-ion battery. There is, however, equivalent lithium content that must be considered. For a lithium-ion cell, this is calculated at 0.3 times the rated capacity (in ampere-hours).
Example: A 2Ah 18650 Li-ion cell has 0.6 grams of lithium content. on a typical 60 Wh laptop battery with 8 cells (4 in series and 2 in parallel), this adds up to 4.8g. To stay under the 8-gram UN limit, the largest battery you can bring is 96 Wh. This pack could include 2.2Ah cells in a 12 cells arrangement (4s3p). If the 2.4Ah cell were used instead, the pack would need to be limited to 9 cells (3s3p).
Restrictions on shipment of lithium-ion batteries
- Anyone shipping lithium-ion batteries in bulk is responsible to meet transportation regulations. This applies to domestic and international shipments by land, sea and air.
- Lithium-ion cells whose equivalent lithium content exceeds 1.5 grams or 8 grams per battery pack must be shipped as "Class 9 miscellaneous hazardous material." Cell capacity and the number of cells in a pack determine the lithium content.
- Exception is given to packs that contain less than 8 grams of lithium content. If, however, a shipment contains more than 24 lithium cells or 12 lithium-ion battery packs, special markings and shipping documents will be required. Each package must be marked that it contains lithium batteries.
- All lithium-ion batteries must be tested in accordance with specifications detailed in UN 3090 regardless of lithium content (UN manual of Tests and Criteria, Part III, subsection 38.3). This precaution safeguards against the shipment of flawed batteries.
- Cells & batteries must be separated to prevent short-circuiting and packaged in strong boxes.
'관련 뉴스 > 2차전지(배터리)' 카테고리의 다른 글
| 바이러스로 배터리 만든다 (0) | 2009.04.03 |
|---|---|
| GM, 차세대 리튬-이온 배터리 계획 발표 (0) | 2009.03.25 |
| Choosing the right battery for industrial applications (0) | 2009.03.19 |
| 하이브리드 자동차용 이차전지기술 (0) | 2009.03.15 |
| 09年锂离子电池实现飞跃 (0) | 2009.03.14 |