Choosing the right battery for industrial applications
Industrial applications have unique power needs and the choice of battery is important. While consumer products demand high energy density to obtain slim and elegant designs, industry focuses on durability and reliability. Industrial batteries are commonly bulkier than those used in consumer products but achieve a longer service life.
Batteries are electro-chemical devices that convert higher-level active materials into an alternate state during discharge. The speed of such transaction determines the load characteristics of a battery. Also referred to as concentration polarization, the nickel and lithium-based batteries are superior to lead-based batteries in reaction speed. This attribute reflects in good load characteristics.
Discharge loads range from a low and steady current flow of a flashlight to intermittent high current bursts in a power tool, to sharp current pulses on digital communications equipment, laptops and cameras. In this paper we evaluate how the various battery chemistries perform in a given application.
What's the best battery for video cameras?
Nickel-cadmium batteries continue to power a large percentage of professional cameras. This battery provided reliable service and performs well at low temperature. nickel-cadmium is one of the most enduring batteries in terms of service life but has only moderate energy density and needs a periodic full discharge.
The need for longer runtimes is causing a switch to nickel-metal-hydride. This battery offers up to 50% more energy than nickel-cadmium. However, the high current spikes drawn by digital cameras have a negative affect and the nickel-metal-hydride battery suffers from short service life.
There is a trend towards lithium-ion. Among rechargeables, this chemistry has the highest energy density and is lightweight. A steep price tag and the inability to provide high currents are negatives.
The 18650 cylindrical lithium-ion cell offers the most economical power source. "18" defines the cell's diameter in millimeters and "650" the length. No other lithium-ion cell, including prismatic or polymer types, offers a similar low cost-per-watt ratio.
Over the years, several cell versions of 18650 cells with different Ah ratings have emerged, ranging from 1.8Ah to well above 2Ah. The cells with moderate capacities offer better temperature performance, enable higher currents and provide a longer service life than the souped up versions.
The typical 18650 for industrial use is rated at 2Ah at 3.60 volts. Four cells are connected in series to obtain the roughly 15 volts needed for the cameras. Paralleling the cells increases the current handling by about 2A per cell. Three cells in parallel would provide about 6A of continuous power. Four cells in series and three in parallel is a practical limit for the 18650 system.
Lithium-ion requires a protection circuit to provide safe operations under all circumstances. Each cell in series is protected against voltage peaks and dips. In addition, the protection circuit limits each cell to a current about 2A. Even if paralleled, the current of a lithium-ion pack is not high enough to drive digital cameras requiring 10 to 15A peak current. Tests conducted at Cadex Electronics have shown that the 18650 allows short current peaks above the 2A/cell limit. This would allow the use of lithium-ion on digital cameras, provided the current bursts are limited to only a few seconds.
What's the best battery for still cameras?
The power requirement of a professional digital camera is sporadic in nature. Much battery power is needed to take snapshots, some with a powerful flash. To view the photo, the backlit color display draws additional power. Transmitting a high-resolution image over the air depletes another portion of the energy reserve.
Most non-professional cameras use a primary lithium battery. This battery type provides the highest energy density but cannot be recharged. This is a major drawback for professional use. Rechargeable batteries are the answer and lithium-ion fits the bill but faces similar challenges to the video cameras.
What is the best battery for medical devices?
One of the most energy-hungry portable medical devices is the heart defibrillator. The battery draws in excess of 10 amperes during preparation stages. Several shocks may be needed to get the patient's heart going again. The battery must not hamper the best possible patient care.
Most defibrillators are powered by nickel-cadmium. nickel-metal-hydride is also being used but there is concern of short service life. In a recent study, however, it was observed that a defibrillator battery cycles far less than expected. Instead of the anticipated 200?cycles after two years of seemingly heavy use, less than 60 cycles had been delivered on the battery examined. 'Smart' battery technology makes such information possible. With fewer cycles needed, the switch to higher energy-dense batteries becomes a practical alternative.
Sealed lead-acid batteries are often used to power defibrillators intended for standby mode. Although bulky and heavy, the Lead-acid has a low self-discharge and can be kept in prolonged ready mode without the need to recharge. Lead-acid performs well on high current spurts. During the rest periods the battery disperses the depleted acid concentrations back into the electrode plate. Lead-acid would not be suitable for a sustained high load.
The medical industry is moving towards lithium-ion. The robust and economical 18650 cells make this possible. The short but high current spurts needed for defibrillators are still a challenge. Paralleling the cells and adding current-limiting circuits that allow short spikes of high current will help overcome this hurdle.
What is the best battery for power tools?
Power tools require up to 50 amperes of current and operate in an unfriendly environment. The tool must perform at sub zero temperatures and endure in high heat. The batteries must also withstand shock and vibration.
Most power tools are equipped with nickel-cadmium batteries. nickel-metal-hydride has been tried with limited success. Longevity is a problem but new designs have improved. lithium-ion is too delicate and could not provide the high amperage. Lead-acid is too bulky and lacks persistent power delivery. The power tool has simply no suitable alternatives to the rugged and hard-working nickel-cadmium.
In an attempt to pack more energy into power tools, the battery voltage is increased. Because of heavy current and application at low temperatures, cell matching is important. Cell matching becomes more critical as the number of cell connected in series increases. A weak cell holds less capacity and is discharged more quickly than the strong ones. This imbalance causes cell reversal on the weak cell if the battery is discharged at high current below 1V/cell. An electrical short occurs in the weak cell if exposed to reverse current and the pack needs to be replaced. The higher the battery voltage, the more likely will a weak cell get damaged.
_________________________
What's the best battery for wheeled and stationary applications?
Consumer products have benefited the most from the advancements in battery technology. The size and weight reductions achieved for the high-end cell phones, PDA's and laptops have not trickled down to batteries for wheeled and stationary applications in an expected fashion. only marginal improvements have been gained on larger batteries. one of the reasons for the apparent lack in progress is the loyalty to the classic sealed lead-acid battery.
The wheeled and stationary industries have several reasons for their unwillingness to change: [1] lead-acid is mature and inexpensive. [2] The low energy density is no major drawback because the battery is either on wheels or is stationary. [3] The limited cycle life can, to some extent, be compensated by using larger batteries. Unlike portable devices, most wheeled and stationary batteries are replaced due to age rather than wear out effect induced by high cycle count.
What's the best battery for wheelchairs?
Wheelchairs and scooters are almost exclusively powered by sealed lead-acid batteries. Regular car batteries are sometimes used for cost reasons. There is, however, a danger of spillage if overturned. Neither are regular car batteries designed for deep cycling. The demanding cycling regiments of wheelchairs and scooters cause an undue strain on these batteries and shorten their lives. nickel-based batteries would be lighter than lead-acid but are more expensive and maintenance prone. Lithium-ion would simply be too delicate, not to mention the high cost.
A new generation of wheelchair is being developed that is able to 'stand up' and climb stairs. These high-tech devices use gyroscopes for balancing. To obtain the extra power needed to run its internal computer and electric motors without adding too much weight, nickel-based batteries are used. The two-wheeled Segway scooter being touted to solve city transportations problems also uses nickel-based batteries.
What's the best battery for the electric bicycle?
Anyone serious about the electric bicycle would use nickel-based batteries. Sealed lead-acid is simply too heavy and does not provide the cycle count needed to satisfy daily use. In addition, lead-acid requires a long charge time of 10 hours and more. Lithium-ion would simply be too expensive and delicate. The lack of a suitable battery that is light, durable and inexpensive is, in my opinion, delaying the public acceptance of the electric bicycle.
What's the best battery for the electric vehicle?
The electric vehicle will gain public acceptance as soon as a battery emerges that is inexpensive and provides 10 years of reliable service. The high cost and limited cycle life of the batteries used in hybrid vehicles negate the savings achieved in burning less fuel. The benefits are more environmental in nature rather than in cost savings. Higher fuel prices could change this equilibrium.
nickel and lithium-based batteries have been tried but both chemistries have problems with durability and stability. lithium-ion has an advantage in weight but this gain is offset by a high price. Similarly, nickel-metal-hydride used for the hybrid vehicle is expensive and requires forced air-cooling. No battery manufacturer is willing to commit to a 10-year warranty. After excursions into new battery chemistries, design engineers always come back to the old but proven lead-acid.
The fuel cell may still be two decades away before offering a viable alternative for cars. An executive from Ford stated recently that the fuel cell may never be feasible to replace the internal combustion engine. Cost and longevity remain major drawbacks.
Since the invention in 1839 by Sir William Grove, the advancements in the fuel cell have been slow. Much attention was then placed on improving the internal combustion engine. It was not until the Gemini and Apollo programs in the 1960s that the fuel cell was used to provide power and water in space. During the 1990s, renewed activities took place and the fuel cell stocks soared. Unlike the rapid developments in microelectronics, which generated income in its early stages, fuel cell research continues to depend on government grants and public investors. It is our hope that one day the fuel cell will become a viable option to the polluting combustion engine.
What's the best battery for stationary applications?
Until the mid 1970s, most stationary batteries were flooded lead-acid. The Valve Regulated Lead Acid (VRLA) allowed batteries to be installed in smaller confinements because the cells could be stacked and mounted in any position. Although VRLA are less durable than flooded lead-acid, simple mounting and lower cost make them the preferred battery system for small and medium sized installations. Most UPS systems repeater stations for cell phones use VRLA. Large installations, such as internet hubs, hospitals, banks and airports still use the flooded lead-acid.
Heat is the main killer of batteries. Many outdoor installations for communication systems lack proper venting, not to mention air conditioning. Instead of the expected 10-year service life, the batteries need replacement after 2 to 5 years. Many batteries in the field are in such bad conditions that they could only provide power for a short time, should a major power outage occur. Stationary batteries are often installed and forgotten.
A Canadian manufacturer of lithium-polymer batteries is taking advantage of the heat problem. They offer lithium-polymer for standby applications, a battery that needs heat to operate. The dry lithium-polymer lacks conductivity at ambient temperature and must be heated. The battery includes heating elements to keep its core temperature at 60°C (140°F). The mains provide the energy for heating. on a power outage, the battery must also provide power for heating the core. To conserve energy, the battery is well insulated. Unlike the VRLA, the high ambient heat does not shorten the lithium-polymer battery. The high cost remains a drawback and only a few lithium-polymer batteries are used for stationary applications today.
Flooded nickel-cadmium batteries have been used for many years in applications that must endure hot and cold temperatures. This battery system is substantially more expensive that Lead-acid but the improved longevity makes up for the higher investment cost. The flooded nickel-cadmium batteries are non-sintered and do not suffer from memory. It should be noted that only the sintered sealed nickel-cadmium cells are affected by memory and need regular discharges.
_________________________
Are the Hybrid Cars here to stay?
The hybrid car is not new - Ferdinand Porsche designed the series-hybrid vehicle in 1898. Called the Lohner-Porsche carriage, the hybrid function served as an electrical transmission rather than power boost. With Mr. Porsche in the driver's seat, the car broke several Austrian speed records, including the Exelberg Rally in 1901. Another example of an early hybrid was the 1915 Woods Motor Vehicle built in Chicago. The car used a four-cylinder internal combustion engine and an electric motor. Below 15 mph (25 km/h), the electric motor propelled the vehicle; at higher speeds, the gasoline engine kicked in to take the vehicle up to a top speed of 35 mph (55 km/h). As part of the Federal Clean Car Incentive Program, Victor Wouk installed a hybrid drive train in a 1972 GM Buick Skylark but the EPA canceled the program in 1976. Meanwhile, Honda and Toyota have made strong headways by commercializing attractive and fuel-efficient hybrid cars.
The hybrid electric vehicle (HEV) conserves fuel by using an electric motor that assists the internal-combustion engine (IC) on acceleration and harnesses kinetic energy during breaking. Furthermore, the IC motor turns off at stops and during slow travel. When full power is required, both the IC engine and the electric motors engage simultaneously to get maximum boost. This power-sharing scheme offers two advantages; it calls for a smaller IC engine and improves acceleration because the electric motor has excellent torque characteristics.
Most HEVs use a mechanical drive train from the IC engine to the wheels. In this respect, the HEV is similar to an ordinary vehicle with crankshaft, clutch and transmission, with the difference of having an electric motor and a battery. This design is known as a parallel configuration. Most up-and-coming plug-in HEVs use the serial configuration in which the wheels are powered by one or several electric motors. Instead of a mechanical link, the IC engine energizes a generator to produce electricity for the motor(s). Similar to a laptop or a cell phone, the driver plugs the car into the AC outlet for an overnight charge. The typical driving range with a full charge is 20 miles or 32 km. on long trips, the IC engine engages to provide continuous power for the electric motors.
What's the best battery for the hybrid car?
The early HEV models used lead acid batteries because there was no alternative. Today, Honda and Toyota employ nickel-metal-hydride (NiMH). This chemistry is lighter and environmentally friendlier than lead-based systems. The battery consists of cylindrical cells that are connected in series to attain several hundred volts. The cell strings are suspended in mid air to allow air-cooling. Figure 1 shows a demonstration pack of an early Toyota hybrid car battery.
|
|
Figure 1: Nickel-metal-hydride battery of a Toyota hybrid car. |
One of the critical battery requirements for hybrid applications is longevity. Rechargeable batteries for consumer products typically last for two to three years. This short service life is no major drawback with cell phones, laptops and digital cameras because the devices get obsolete quickly. At $2,000 to $3,000 per battery pack, the replacement cost of an HEV battery would constitute a major expense.
Most batteries for HEV are guaranteed for eight years. To meet this long service life, the cells are optimized for longevity and not size and weight, as is the case with portable applications. Since the battery runs on wheels, the increased weight and size is not too critical.
A NiMH for an HEV can be charged and discharged 1,000 times if done at an 80% depth-of-discharge. In a hybrid vehicle, a full discharge occurs seldom except if the owner lives on a mountain and requires all available battery power to commute home. Such a routine would add stress to the battery and the life would be shortened. In most other application, the hybrid car only uses 10% of the rated battery capacity. This allows thousands of charge/discharge cycles. Batteries in satellites use a similar system in which the battery discharges less than 10% during a satellite night. NASA achieves this by over-sizing the battery.
One of the limitations of NiMH is moderate energy conversion efficiency. This translates to the battery getting hot on charge and discharge. The charge efficiency is best at 50-70% state-of-charge. Above 70% the battery cannot absorb the charge well and much of the charging energy is lost in heat. Operating a battery with a partial charge requires a larger mass that lowers the energy-to-weight ratio and efficiency.
The Japanese car manufacturers have tried several battery chemistries, including going back to lead acid. Today, the focus is on lithium-ion. The cobalt-based lithium-ion is one of the first chemistries in the lithium family and offers a very high energy density. Unfortunately, this battery system cannot deliver high currents and is restricted to portable applications.
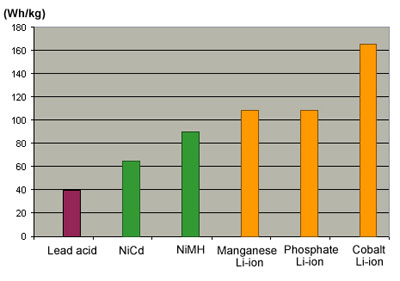
HEV manufacturers are experimenting with manganese (spinel) and phosphate versions. These lithium-ion systems offer an extremely low internal resistance, deliver high load currents and accept rapid charge. Unlike the cobalt version, the resistance stays low throughout the life of the battery. To verify the characteristic of manganese-based lithium-ion, a research lab applied 30,000 discharge/charge cycles over a period of seven years. Although the capacity dropped from 100% to 20%, the cell retained its low internal resistance. The drawback of manganese and phosphate is lower energy density but these systems provide 20% more capacity per weight than NiMH and three times more than lead acid. Figure 2 illustrates the energy densities of the lead, nickel and lithium-ion systems.
(See also http://www.batteryuniversity.com/partone-5A.htm) It should be noted that lithium-ion systems have the potential of higher energy densities but at the cost of lower safety and reduced cycle life.
|
|
Figure 2: Energy densities of common battery chemistries. |
The Lithium-ion systems are promising candidates for both the HEV and plug-in HEV but require more research. Here are some of the roadblocks that need to be removed:
Durability: The buyer requests a warranty of ten years and more. Currently, the battery manufacturer for hybrid electric vehicles can only give eight years on NiMH. The longevity of lithium-ion has not yet been proven and honoring eight years will be a challenge.
Cost: If the $2,000 to $3,000 replacement cost of a nickel-metal-hydride pack is prohibitive, lithium-ion will be higher. These systems are more expensive to produce than most other chemistries but have the potential for price reductions through improved manufacturing methods. NiMH has reached the low cost plateau and cannot be reduced further because of high nickel prices.
Safety: Manganese and phosphate-based lithium-ion batteries are inherently safer than cobalt. Cobalt gets thermally unstable at a moderate temperature of 150°C (300°F). Manganese and phosphate cells can reach 250°C (480°F) before becoming unsafe. In spite of the increased thermal stability, the battery requires expensive protection circuits to supervise the cell voltages and limit the current in fail conditions. The safety circuit will also need to compensate for cell mismatch that occurs naturally with age. The recent reliability problems with lithium-ion batteries in portable devices may delay entry into the HEV market.
Availability: Manufacturers of manganese and phosphate cells can hardly keep up with the demand. A rapid increase of lithium for HEV batteries would put a squeeze on battery production. With 7 kg (15 lb) of lithium per battery, there is talk of raw material shortages. Most of the known supplies of lithium are in South America, Argentina, Chile and Bolivia.
The plug-in hybrid electric vehicle (PHEV)
Imagine a plug-in electric vehicle that can go 20 miles (32 km) with a single charge from the electrical outlet at home. There is no pollution and the neighbors won't hear you coming and going because the vehicle is totally silent. With the absence of gas tax, the road system is yours to use for free. Or is it?
As good as this may sound, the savings will be small or non-existent because of the battery. Dr. Menahem Anderman, a leading expert on advanced automobile batteries, says that we still have no suitable battery for the plug-in HEV and that the reliability of lithium-ion technology for automotive applications has not yet been proven. Unlike the ordinary HEV that operates on shallow charges and discharges, the plug-in HEV is in charge depletion mode that requires deep discharges. To obtain an acceptable driving range, the PHEV battery will need to be five times larger than the HEV battery. With an estimated life span of 1000 full charge and discharge cycles, the battery would need to be replaced every three years. At an estimated $10,000 per battery replacement, the anticipated cost savings would be quickly exhausted.
Modern cars do more than provide transportation; they also include auxiliary devices for safety, comfort and pleasure. The most basic of these auxiliaries are the headlights and windshield wipers. Most buyers would also want heating and air-conditioning systems. These amenities are taken for granted in gasoline-powered vehicles and will need to be used sparingly in a PHEV.
Analysts give another 10 years before a viable plug-in HEV will be available. The promise of a clean-burning fuel cell car is still vivid in our memory. Analysts now estimate 20 years before the fuel cell is ready for mass-produced cars. There are rumors that the fuel cell may never make it into an ordinary car. If this is true, a dream will go down in history with the steam-powered airplane of the mid 1800s that was simply too cumbersome to fly.
The paradox of the hybrid vehicle
At the Advanced Automotive Battery Conference in Hawaii, a delegate member challenged a maker of HEVs with the claim that a German diesel car can get better fuel economy than the hybrid. The presiding speaker, being a trained salesman, flatly denied this notion. There is some truth to his claim, however. on the highway, the diesel car is indeed more fuel-efficient but the HEV has the advantage in city driving. Power boost for fast acceleration and regenerative breaking are advantages that the German diesel does not offer.
Someone then asked, "What would happen if the HEV depletes its batteries while driving up a long mountain pass? Will the car have enough power?" The answer was that the car would make it with the IC engine alone but the maneuverability would be restraint. To compensate for this eventuality, some HEV manufacturers offer SUVs featuring a full-sized IC motor of 250 hp and an electrical motor at 150 hp; 400 hp in total. Such a vehicle would surly find buyers, especially if the government provides grant money for being 'green.' It's unfortunate that the buyers of a small car or the commuters taking public transport won't qualify for such a handout.
Conclusion
We anticipate that lithium-ion will eventually replace nickel-metal-hydride in hybrid electric vehicles but short service life, high manufacturing costs and safety issues will stand in its way today. We need to remind ourselves that the automotive market can only tolerate a marginal cost increase for a new battery technology. In terms of added capacity, lithium-ion offers only a 20% increase in energy density per weight over nickel-based systems. The nickel-metal-hydride has proven to work well in current HEVs and a new chemistry would need to offer definite advantages over present systems to find buyers.
Toyota, Honda and Ford are leading in HEV technology. Other major automakers are expected to offer competitive models by 2010. Currently, Panasonic EV Energy and Sanyo supply over 90% of the HEV batteries. Both companies are also developing lithium-ion batteries.
While Japan and Korea are focusing on manganese systems, the USA is experimenting with phosphate, the chemistry that made the A123 Systems famous. Europe is relying on clean-burning diesel. These engines are so clean that they won't even stain a tissue that is placed on the exhaust pipe. BMW is working on a zero emission hydrogen car.
Time will tell who will be the winner in the race for cleaner, more fuel-savvy vehicles and longer-living cars. In terms of longevity, the diesel would be the winner today. We hope that future batteries will one-day have the endurance to match or exceed the robust diesel engine.
References: Menahem Anderman, Status and Prospect of Battery Technologies for Hybrid Electric Vehicles,
including Plug-in Hybrid Electric Vehicles, January 2007.
'관련 뉴스 > 2차전지(배터리)' 카테고리의 다른 글
| GM, 차세대 리튬-이온 배터리 계획 발표 (0) | 2009.03.25 |
|---|---|
| What's the best battery? (0) | 2009.03.19 |
| 하이브리드 자동차용 이차전지기술 (0) | 2009.03.15 |
| 09年锂离子电池实现飞跃 (0) | 2009.03.14 |
| 10초만에 휴대폰 충전 가능한 배터리 소재 기술 개발 (0) | 2009.03.12 |